

INano™ Optimux
多功能连续纳米药物制备系统(GMP)

INano™ L/L+
快速纳米药物制备系统

INano™ S
大规模商业化纳米药物制造系统

INano™ X
快速纳米药物制备系统

细胞转染试剂盒

应用试剂盒

器官靶向试剂盒

验证试剂盒

DNA试剂盒

蛋白试剂盒
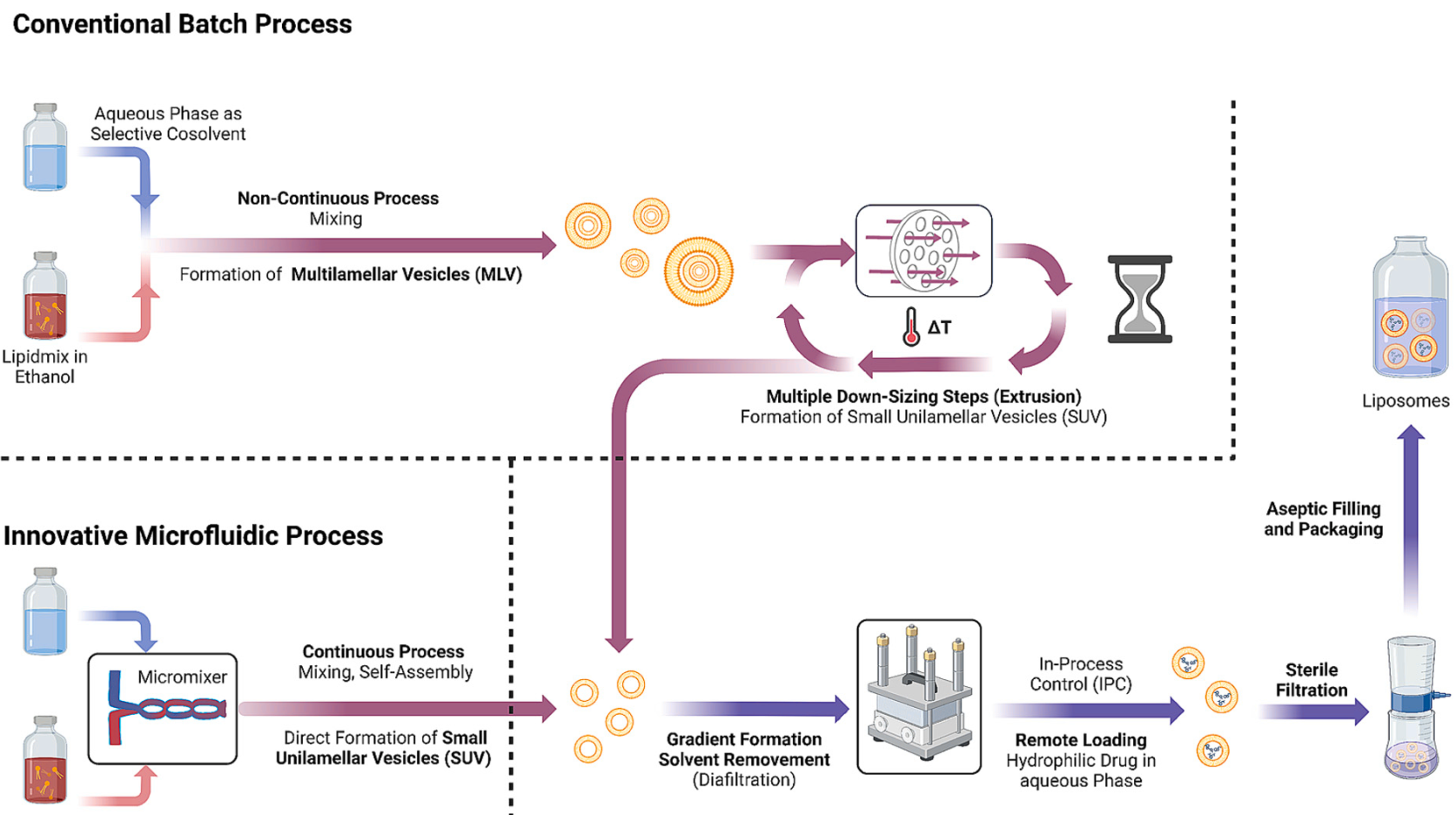
Fig. 1. Schematic comparison of the conventional liposome production method using the batch process with MLV production followed by extrusion steps (top) for downsizing and the microfluidic approach presented here (bottom) using a micromixer (instead of extruder) in which the desired nanoliposomes are produced by one step and without the need to work above the lipid phase transition temperature.
Table 1 Comparison of the technical parameters of all tested micromixers for continuous liposome fabrication.
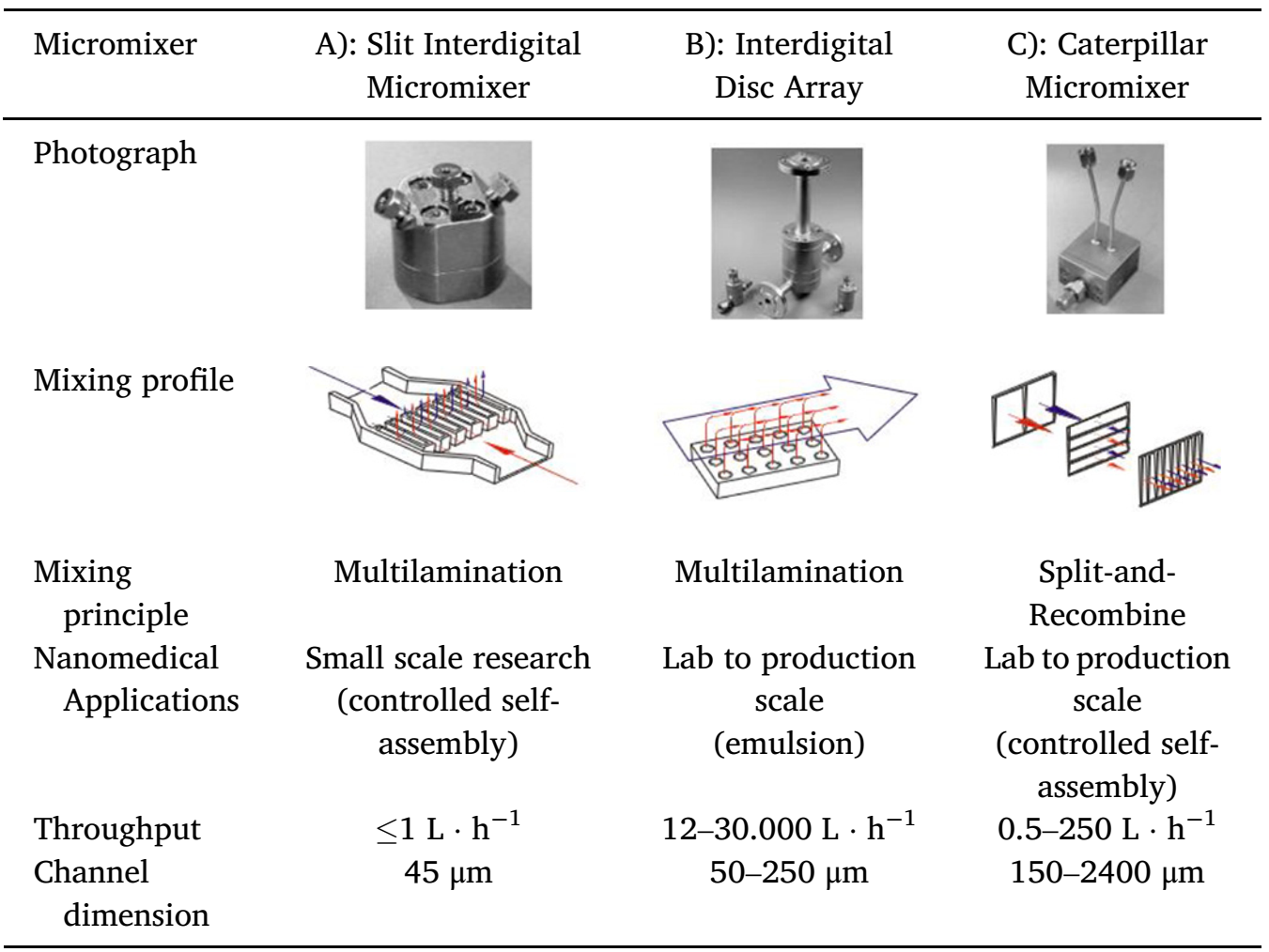
Table 2 Comparison of physicochemical characteristics of nanoliposomes with ammonium sulfate gradient obtained with different micromixers at various flow rate scales

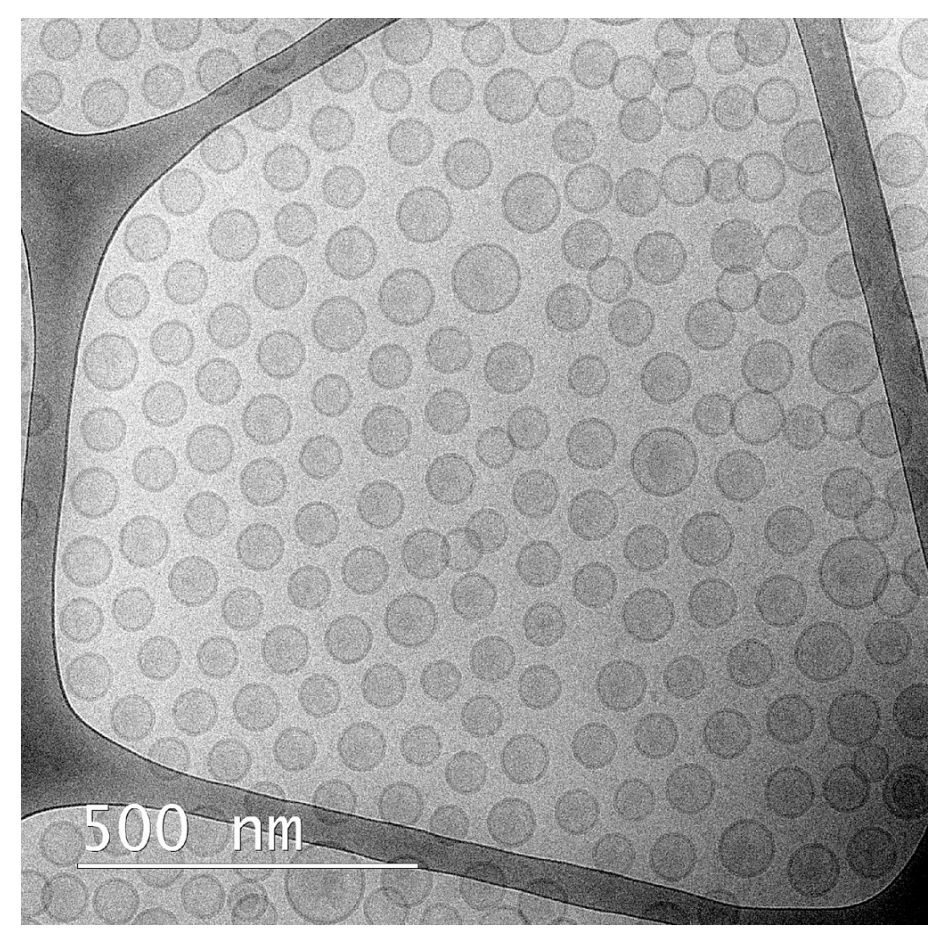
Fig. 2. Cryogenic Transmission Electron Microscopy (cryo-TEM) image of continuously manufactured liposomes with CAT300 micromixer before DXR remote loading as representative example.
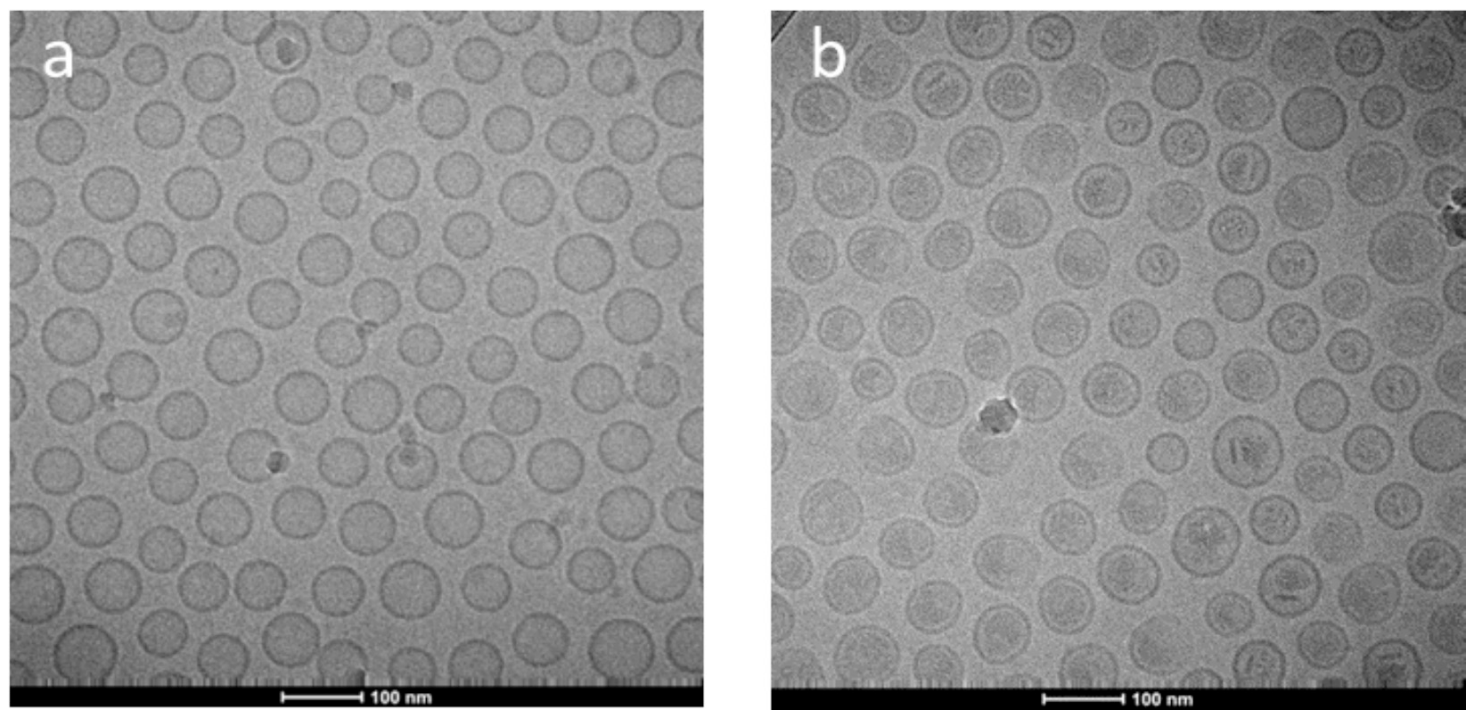
Fig. 3. Cryogenic transmission electron microscopy images of continuous manufactured liposomes before (a) and after (b) remote loading with MPS.
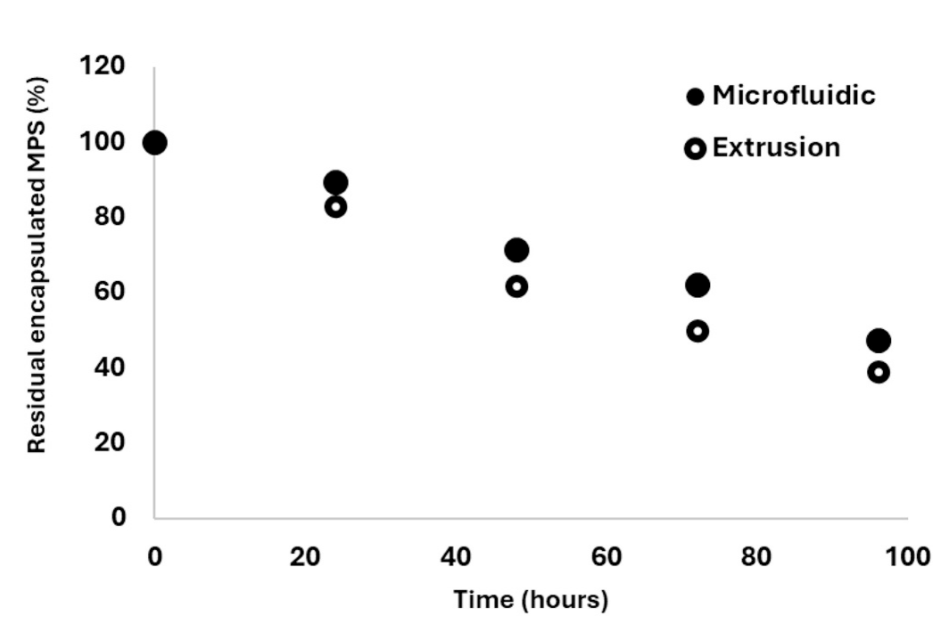
Fig. 4. Comparison of release kinetics (“dissolution” assay) of MPS at 37 ◦C in human plasma from nanoliposomes produced using a microfluidic-based process and conventional extrusion.
对于阿霉素(DXR)负载的脂质体,药物负载效率达到95%以上,与挤压法制备的脂质体相当(表3)。与市售的挤出法制备的制剂相比(图6),特别是Doxil®类似物,微流控法制备的脂质体在轴向比(elongatedness)和形状分布上表现出更高的均一性(表4)。
Table 3 Physicochemical characterization of liposome samples with calcium acetate gradient directly after formulation, after diafiltration and after remote drug loading with MPS. Size, size distribution and Zeta potential were measured using a Zetasizer nanoZS.
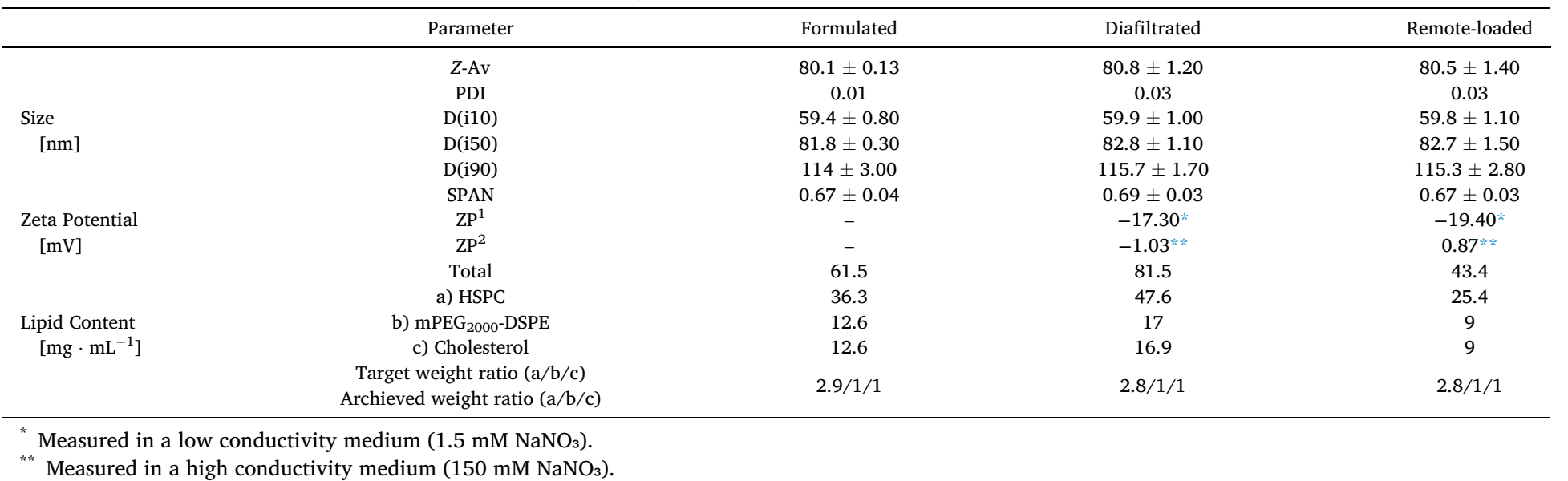
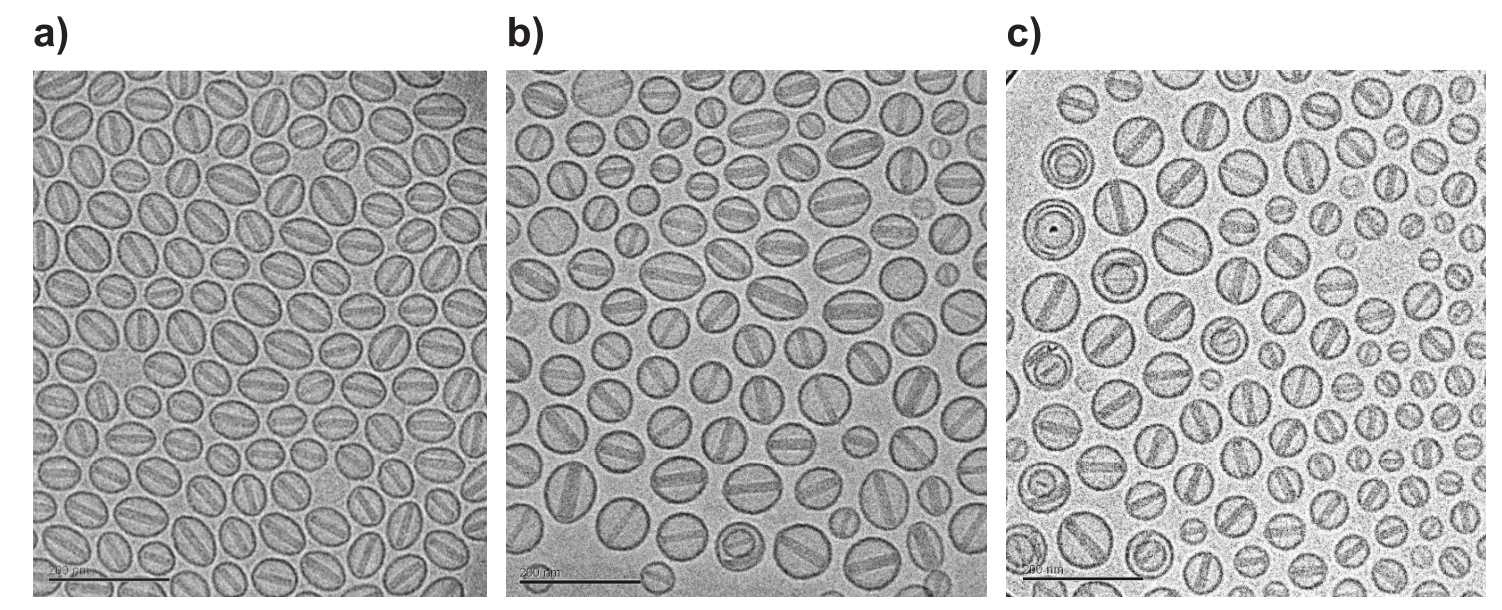
Fig. 6. CryoTEM images of Doxil®-like liposomes prepared by a) Fraunhofer IMM using microfluidics, further processed, and loaded with Doxorubicin by Ayana Pharma; b) Caelyx® and c) Ayana Pharma starting with stepwise extrusion downsizing. Scalebar 200 nm.
Table 4 Cryo-TEM comparison of Doxil®-like liposomes prepared with either microfluidics or extrusion.Analysis was done by measurement of multiple images with a total of N > 1000 particles as representative sample according to reference

Table 5 Thermodynamic parameters and size distribution of nanoliposomes with calcium acetate transmembrane gradient produced using a microfluidic based process and conventional extrusion.

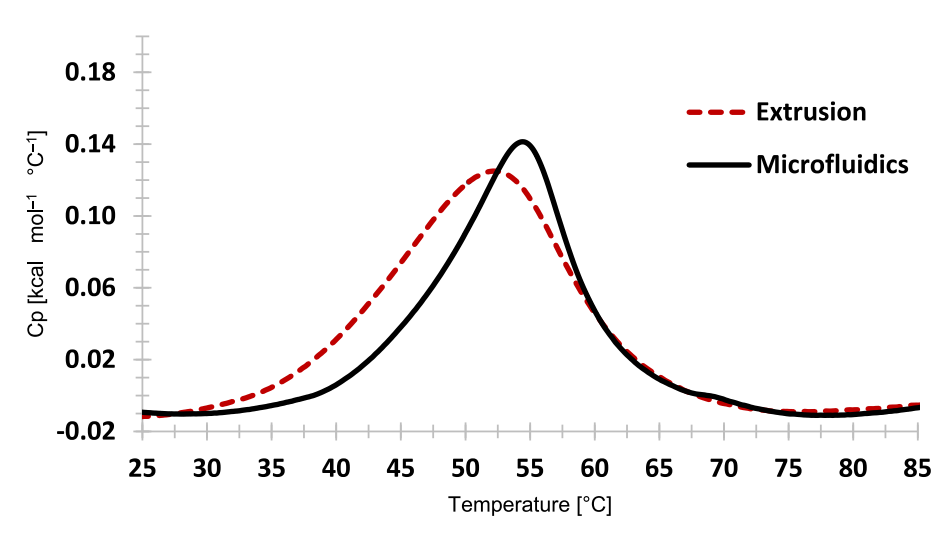
Fig. 5. DSC thermograms of nanoliposomes with calcium acetate transmembrane gradient produced using a microfluidic based process and conventional extrusion
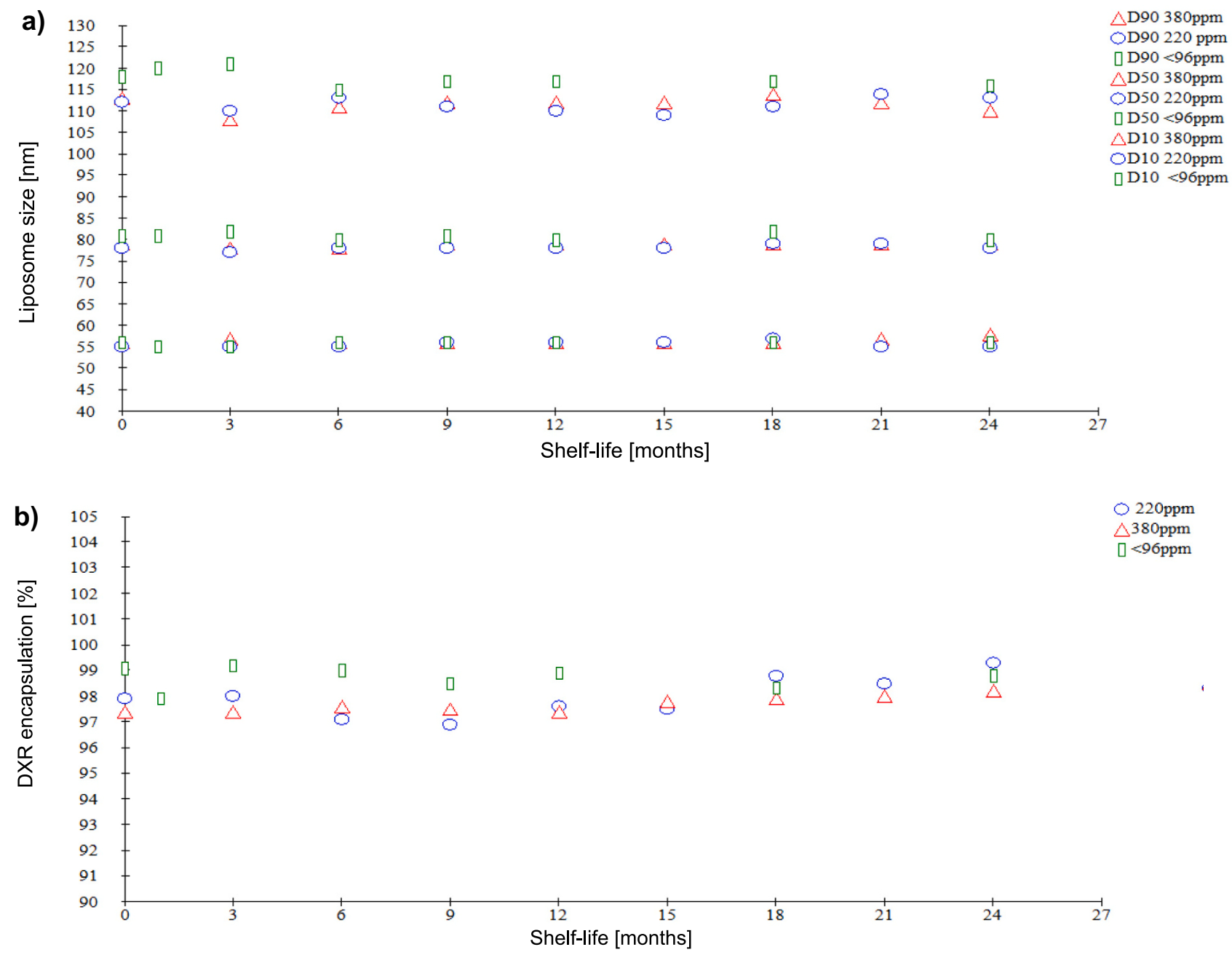
Fig. 7. a) Independence of liposome size distribution from the ethanol content in the range from below 96 ppm to up to 380 ppm. These Parameters were followed up for 24 months at 5◦ ± 3 ◦C. b) Independence of extent of doxorubicin encapsulation from ethanol content in the range from below 96 ppm to up to 380 ppm. These Parameters were followed up for 24 months at 5◦ ± 3 ◦C.
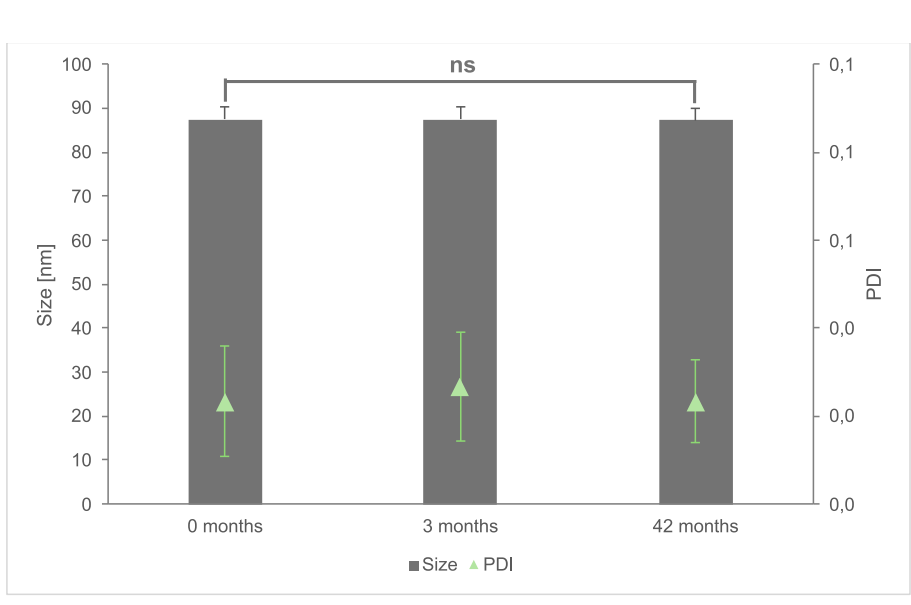
Fig. 8. Long-term stability of microfluidically prepared liposomes (three individual production runs) that comply with FDA guidelines for ethanol content of <100 ppm. DLS-Data was analyzed using One way ANOVA showing no significance regarding changes in size and PDI during the entire observation period.
参考文献:Comparing continuous micromixing and extrusion downsizing for PEGylated nanoliposomes remotely loaded with doxorubicin or the steroid pro-drug methylprednisolone hemisuccinate. https://doi.org/10.1016/j.jconrel.2025.113707
